Concrete hardeners – facts and myths
There are two ways to harden the surface of concrete: mechanically or chemically. In the production of industrial concrete floors, practice shows that both methods are often used simultaneously to optimize the surface’s functional properties.
DRY SHAKES AND LIQUID HARDENERS FOR CONCRETE
The first method of hardening concrete involves troweling in a powdered mixture, known as a “dry shake,” into the concrete surface. Chemical hardening of concrete surfaces, on the other hand, involves applying liquid mineral compositions. The first generation of liquid concrete hardeners includes sodium, magnesium, and potassium silicates. In the 1990s, lithium silicates emerged, primarily because of their alleged ease of application. The third-generation product is colloidal silica. Many myths have arisen around the chemical hardening of concrete, often spread by the manufacturers themselves. Taking a closer look at this technology is worth it to fully exploit the opportunities it offers contractors and investors.
CHEMICAL REACTION WITHOUT COATING THE PORES
The principle of operation of liquid concrete hardeners is the same. Any manufacturer representative will tell us that “our product X, or rather the silica it contains, reacts with the by-product of cement hydration, i.e. calcium hydroxide, to form calcium silicate hydrate (C-S-H). As a result, additional C-S-H phases (gel) are formed in the pore space, which reduces porosity, strengthens the structure, and increases the durability of the concrete surface.” This is true. All aqueous silicate solutions and colloidal silica work in a similar way. They reduce the surface porosity of concrete by densifying the structure of gel pores and capillaries. (hence the term “densifiers”).
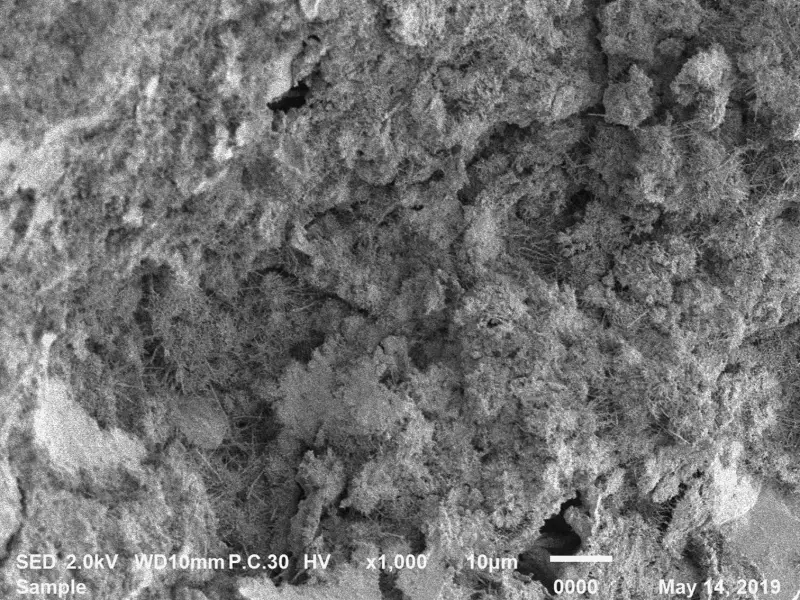
Photo: CONCRETE SURFACE BEFORE AND AFTER APPLICATION OF COLLOIDAL SILICA

CHEMICAL HARDENERS – THE TRUTH ABOUT SILICATES
Unlike typical impregnants described in the PN-EN 1504-2 standard, silicates are in most cases inorganic compounds. Many years ago, I was involved in the distribution of a world-renowned sodium silicate product called Ashford Formula. During training, we were told that the marbled shine that the floor gains over time after application of the preparation is due to the increasing hardness of the surface and the unique action of crystalline silica arranged at the molecular level. It turns out that the truth is different.
All silicates form a silica layer on the surface of concrete. The use of lithium, potassium, or sodium silicate on a smooth surface leads to the formation of a thin silica layer, which, under the influence of movement and friction, begins to reflect light better from the concrete surface over time (hence the slogan “the older it gets, the better it looks”). . This is obvious because the texture of the slab surface is reduced without the use of abrasives. Therefore, most hardeners can be smoothed to achieve a glossy finish. This layer is not very thick, but it has good abrasion resistance and polishes well. Silica has a hardness of 7 on the Mohs scale. Naturally, this layer will also wear out over time as a result of abrasion. To confirm this thesis, it is sufficient to conduct a roughness test on the surface of untreated concrete and concrete treated with silicate. In particular, concrete hardened with high-viscosity silicate and high solid content will have low surface roughness.
HOW TO EFFECTIVELY STRENGTHEN CONCRETE FLOORS
When using liquid hardeners for concrete, two important rules must be followed. Firstly, they should not be used on freshly troweled concrete. The essence of the chemical reaction is the binding of calcium hydroxide in concrete, and the longer the concrete matures, the more cement hydration by-products are produced. The application of silicates followed by covering the concrete with foil usually results in unsightly efflorescence on the floor surface.
Secondly, more is better. Liquid concrete hardeners should be used to fully saturate the pores. Silicates, both lithium and sodium, are more difficult to apply because their high pH and high viscosity make it difficult for them to penetrate the substrate and require a lot of attention and care during application. This is not the case with colloidal silica. Nanosilica penetrates perfectly even into concrete hardened with surface sprinkles. In addition, it is more reactive, easier to apply, and creates hydrophilic (water-loving) concrete floor surfaces.
Author: Marek Szczerbowski
See also